EXPERT INSIGHT
A 5-Step Approach to Leveraging 3D Technology for Medical Device Start-Ups

The medical device market is very innovative and dynamic. Many new players enter the field each year, covering a range of medical applications from personalized ankle implants and tricuspid valve replacement devices to applications as diverse as bioresorbable implants.
As a start-up in this field of medical devices, many challenges will cross your path. There needs to be more than a novel idea for a complex treatment because, as a start-up, you must differentiate yourself and stay ahead of the rapidly growing competition. Moreover, you need to convince investors and clinicians of your technology. Another major challenge is to move quickly to reach patients faster and increase your clinical coverage and market share.
This article details a 5-step approach for start-ups to use 3D technology to overcome these challenges and rapidly and successfully bring your medical device to the market.
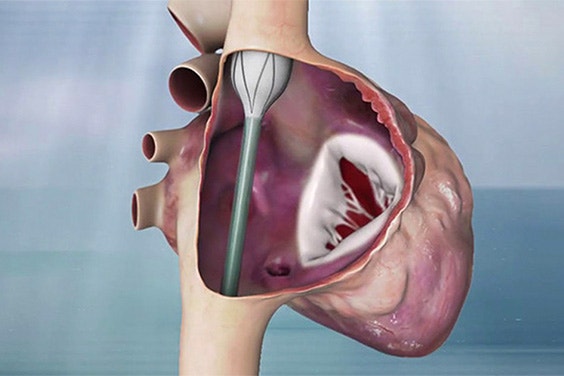
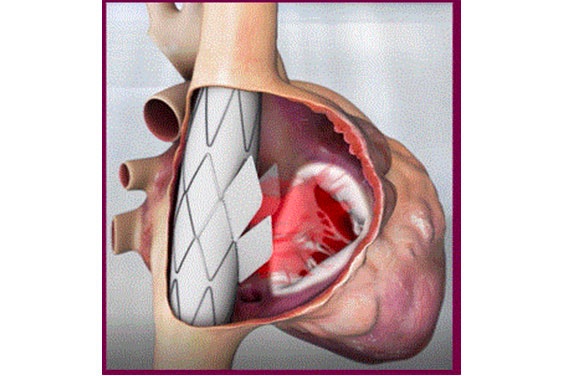
Innoventric brought their forward-thinking solution to treat tricuspid regurgitation (TR) from idea to realization more quickly.
From a great idea to an even better prototype
All start-ups have one thing in common: start with a great idea. In the medical device world, this idea often formed from a clear clinical need and might even have originated from a collaboration with a clinician. With this great idea, new questions arise: what is the target population? What’s the optimal device shape? What size is needed? And are multiple sizes required to cover the population? 3D technology has proven to provide an accurate understanding of the anatomy of the target population and can be used as a reliable source for design input.
The first version of the prototype will most likely be different from the version that will ultimately reach patients. In almost all cases, design iterations to the prototype are vital. Here, the main benefit of 3D technology is that it allows for quick pinpointing of potential design issues and the required design iterations. This can be, on the one hand, thanks to 3D-printed benchtop models of realistic test scenarios (ranging from specific patients). On the other hand, it can be thanks to in silico clinical trials where digital twins are used to simulate a particular treatment and pinpoint potential device failure. Once the required design iterations are completed, and the prototype has passed all verification tests, you can move forward to animal and human trials with more confidence in your device.
Some examples of start-ups that have benefited from 3D technology to optimize their device are 4C Medical, CADSkills, and Innoventric.


Make it work for the first patient
The end goal of any medical device start-up is to reach as many patients as possible and provide the best possible care. Here, the saying “from one to one million” can be taken literally as the successful treatment of one million patients often starts with one first successful procedure. This first patient needs to be selected very carefully. You want to make sure that the anatomy of this patient is suitable for your device, that the requirements of the study protocol are met, and that any critical measurements are fulfilled. In this screening process, 3D technology can play a critical role. Understanding the anatomy of this first patient in 3D, performing these critical measurements on accurate 3D models, and ultimately deciding whether it’s a good fit.
The planning and design stage starts once a patient has passed the screening stage. Here, it depends on the specific medical application. For example, for personalized orthopaedic implants, you need a design that fits specifically to this patient. While for structural heart therapy, the device is typically a standard size, but the patient-specific planning makes this a personalized treatment.
Some examples of start-ups that have benefited from 3D technology to successfully treat their first patients are 4C Medical, CADSkills, Innoventric, and restor3d.


Build evidence for stakeholders
Along the way, multiple types of stakeholders will need to be convinced that your device stands out compared to the competition. First of all, investors will need to be convinced to financially support your start-up in order to fund all required steps to bring your device to the market. As the rounds of investment succeed each other, each time, more evidence will need to be brought to the table to continue convincing investors. At first, investors will need to be intrigued by your concept and believe in the idea; afterwards they will want to see some proof that the prototype is working properly, and eventually they will want to see clinical evidence.
Secondly, clinicians will need to be convinced that your device solves a clinical need, performs well, is an improvement compared to what they’re doing now, and is safe and intuitive to use. A third group of stakeholders are the regulatory bodies, whom you will have to provide with substantial evidence to start your clinical trial and ultimately to get market approval.
Even though those different types of stakeholders differ, they share the same need for evidence. 3D technology has shown a very suitable method for building this evidence.
Some examples of start-ups that have benefited from 3D technology to build evidence for stakeholders are 4C Medical and restor3d. Other interesting references on this topic are the podcast with Thierry Marchal from Ansys and the webinar with Pras Pathmanathan from the FDA.
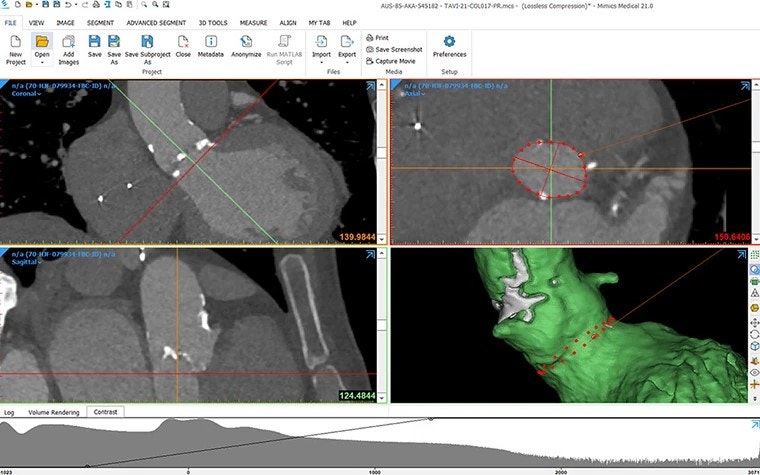

Efficiently scale up as the number of cases increases
In their journey from one to one million patients, start-ups must adapt according to the growing number of cases. Efficiently managing this scale-up, while optimizing the training of new employees and ensuring consistent results, is a versatile challenge all start-ups face in the scale-up phase.
3D technology, combined with smart use of AI and automation techniques, can be one way of overcoming these challenges. Both artificial intelligence and automation through scripting allow for a critical time gain on repetitive tasks, can reduce the learning curve for new employees, and increase the consistency of the results.
Another route — that can be taken in parallel — to overcome these scale-up challenges is to organize your case management more efficiently. Simple Excel files, PDF reports, and communication via mail will do for the first few patients but will quickly become a bottleneck when the number of cases increases. Apart from structural changes to more efficiently follow-up on all cases, this will also improve the traceability of your devices and ameliorate the communication inside your medical start-up ànd with the clinical team in the hospitals.
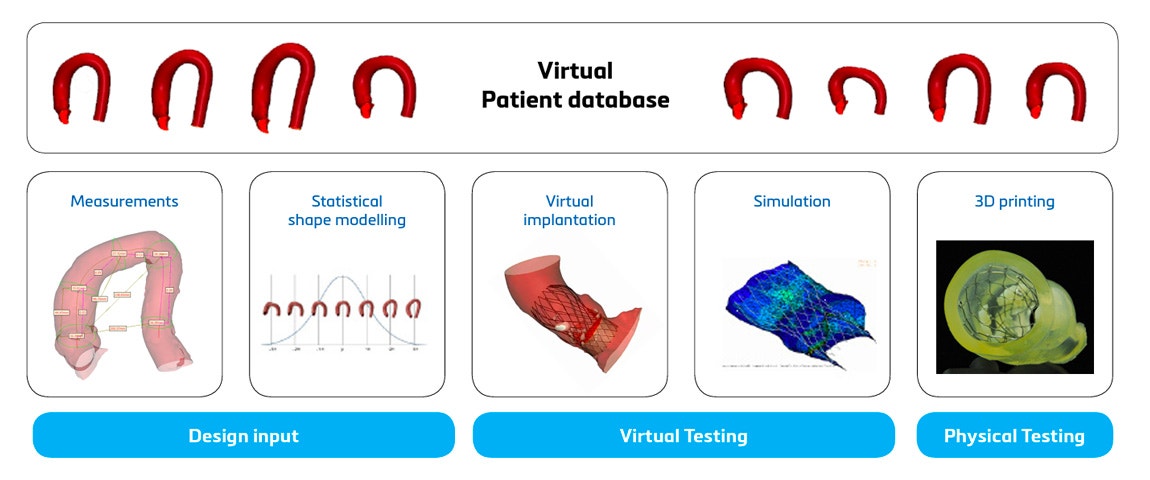
Safeguard the return on investment
Although 3D technology requires an initial investment, it pays off at multiple stages of the journey. First of all, the use of benchtop models and digital twins leads to reduced R&D costs thanks to the reduced amount of (expensive) cadaver labs and the reduced amount of R&D labor hours and hence costs.
Secondly, thanks to 3D technology, a medical device can faster reach the market and hence increase the market share. This leads to increased revenue. Thirdly, using digital twins for your medical device development can reduce risks. Not only by pinpointing potential device failure early on and therefore avoiding device failure later down the line but also by enabling you with the precise knowledge and confidence of what population you can cover with which sizes (or not).
Some examples of start-ups that have benefited from 3D technology to safeguard the return on investment are Ossiform, the examples given in this article about digital twins, and the e-book on virtual patients.
L-103493-01
Share on:
You might also like
Never miss a story like this. Get curated content delivered straight to your inbox.
