EXPERT INSIGHT
Start Leveraging Digital Twins for Your Medical Device Design with 5 Key Questions
“Digital twins and in silico medicine are the most promising solution to refine, reduce, and (partially) replace animal testing and clinical trials through in silico trials. Eventually, digital twins have the potential to predict and prevent diseases making our lives longer and healthier,” said Thierry Marchal, Chief Technologies Healthcare for EMEA at Ansys and Secretary General of the Avicenna Alliance. Although the benefits are well supported, and this technology is quickly becoming the norm in medical device development, there is still some skepticism in the market. This article aims to answer these lingering doubts and reflect on enhancing your medical device design by implementing digital twins.
A digital twin is a computational model representation of at least one physical patient that allows you to optimize the treatment for that patient. This link can be used once or continuously. The digital twin can learn and be updated from multiple sources, representing an individual or a whole patient population with a particular disease, ethnicity, age, or gender. It also allows analysis to help anticipate problems and plan for the future. The term digital twins refers to a specific subsegment of another widely used term, virtual patients.
Digital twins are transforming healthcare at many levels — from groundbreaking academic research to accelerating medical device design to predictive planning for clinical cases. With the following five questions, you’ll have a full consideration of how to integrate digital twins into your medical device design and operation processes.
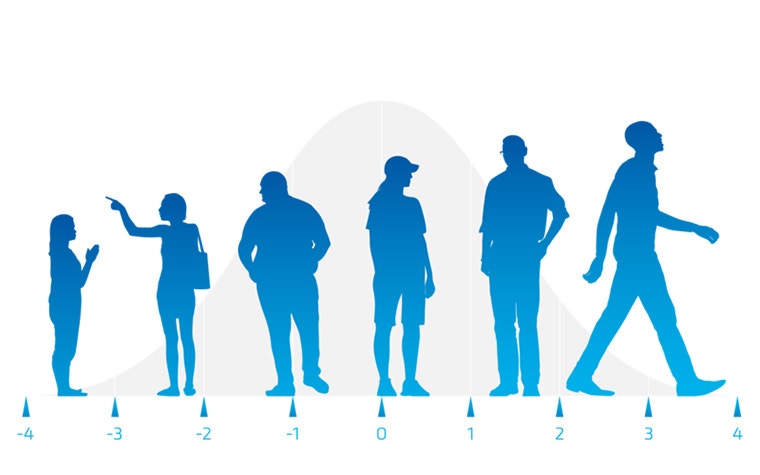

1. What are the benefits and limitations of using digital twins?
At the start of the product life cycle, digital twins play a crucial role in gathering relevant design input. Analyzing individual patients and statistical shape models representing the average and variation enhances the anatomical understanding of a specific population and its coverage. This leads to improved device design with an optimized shape that better fits the population. It could reduce the number of sizes needed to cover the patient population.
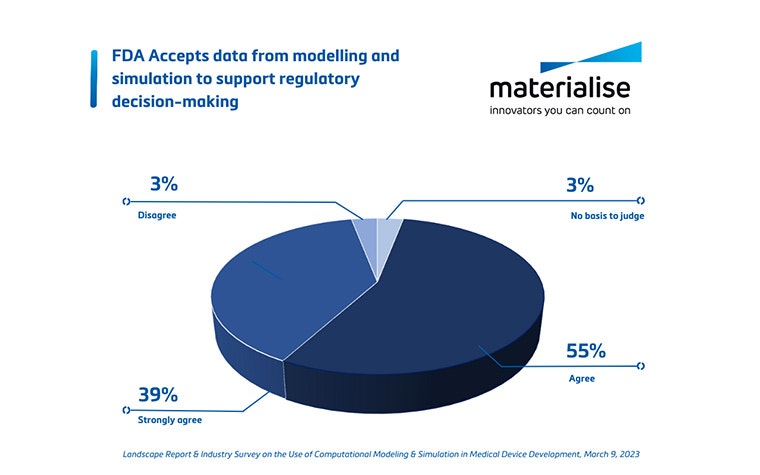
When a prototype has been designed, digital twins can support you in the verification and validation (V&V) step. Virtual and physical tests can be performed on accurate replicas of patients or, again, on a statistical shape model representing the average patient. They can also be adapted to review extreme cases. This is critical to pinpointing potential device failure as early as possible, making quick digital design iterations if needed, and moving forward to clinical trials with more confidence in your device and a reduced risk of post-market device failure. Carrying out the V&V using digital twins can help build a stronger case for regulatory submissions and can lead to a cost reduction and a faster commercial launch (see question 3).
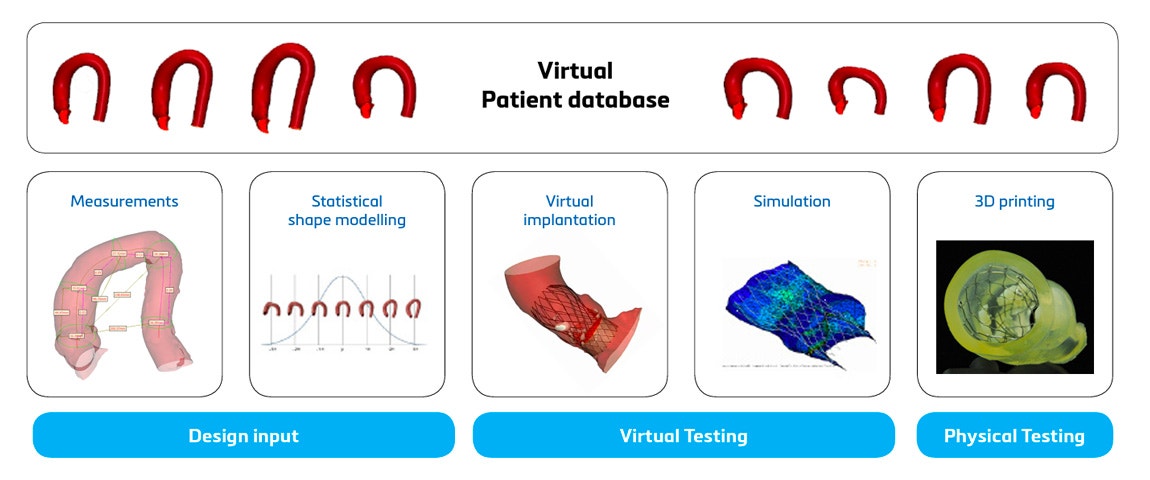
Apart from the above advantages, there are also a few limitations when using digital twins. Understanding these to make full use of this technology is critical. The most important restriction is that there are R&D projects that are unsuitable to benefit from this technology (see question 2). A second limitation is that using digital twins requires a disruptive change (see question 4). Thirdly, for many lacking some experience with computational modeling and simulation (CMS), this technology is often seen as a vast black box, and there is a misconception that only a lucky few understand the complexity and can use it. This is a misunderstanding; all these concerns will be addressed (see question 5). Lastly, the clinical field has shown some resistance to accepting digital twins, leading to slower adoption despite the enthusiastic deployment in some hospitals. For further reference, you can listen to the Medical 3D Players Podcast with Thierry Marchal: Medical 3D Players: How Can Computer Simulation Save Lives?
2. What would be a suitable type of medical device development project?
As mentioned, not every medical device development project is suitable to benefit from the use of digital twins. The first round of questions you should ask yourself to define if your project is worth the investment is:
- Will you work on a device implanted in complex anatomy?
- Is anatomical understanding and fit key to the success of your device?
- Is there a long and costly path to bring this innovative idea to the market (through regulatory clearance)?
If the answer is yes to all three questions, you should consider digital twins as it can aid in increasing patient safety while reducing time and cost to market.
A second round of questions is about what phase of your R&D project you are in:
- Are you in the beginning phase, where you’re gathering relevant design input to decide on the device shape, size, number of sizes, etc.?
- Are you a bit further down the line and already creating a realistic test model to validate your device before moving to animal and human trials?
- Or is your device already in clinical trials or commercially launched, but device failure occurred and forced you to urgently work on a redesign?
If either of the three questions applies to your R&D project, you’re at one of the product life cycle stages where digital twins have proven to shine. The earlier you start your process, the biggest will be the benefits and return on investment (ROI).
Some examples of R&D projects that tick the boxes of both appropriate characteristics and phase are defining the design input for a novel personalized ankle implant, testing a new tricuspid replacement device in a realistic test set-up, and performing a redesign on a hip implant that failed in a high percentage of clinical cases.
3. How can you calculate the return on investment?
Financial benefits vary from company to company, making it almost impossible to calculate one value for all companies' return on investment (ROI). However, the financial benefits can be generalized into three categories:
- First, implementing digital twins as a standard R&D tool can reduce the time to the market. On one hand, having spot-on device documentation saves time for regulatory bodies. On the other hand, a faster commercial launch leads to earlier sales results, increased market share, and increased revenue. In the 3D Players Podcast, Thierry Marchal formulated this very nicely: "Rather than doing a lot of clinical trials, we can create some digital evidence resulting from simulations before the clinical trial. That means that we are communicating a lot of evidence before implanting a device in a real patient.” Thierry also mentions an example of how Medtronic saved two years and over 10 million euros by using digital twins instead of a traditional clinical trial to answer additional questions from the FDA (as elaborated in this report).
- Secondly, the use of digital twins can reduce R&D costs. This might seem contra-intuitive initially as it requires additional investment, but it brings cost savings in other domains. Think of the expensive R&D labor costs that are significantly reduced because of the quick answers digital twins can provide to ‘What does my average patient population look like and how can I quantify it?’, ‘What sizes should my device have to cover 90% of this population?’ and ‘What does an extreme case look like, how can I get a dataset of that, and is my device suitable for such a case?’. Besides reduced labor costs, spending on traditional V&V techniques also diminishes. These realistic test set-ups resulting from digital twins offer a great alternative to the more expensive cadaver lab testing and allow pinpointing potential device failures before going to more expensive animal and even human trials, potentially reducing the number of cases required. Lastly, an R&D opportunity cost is associated with the cross-pollination for other R&D projects that can benefit from digital twins.
- Thirdly, leveraging digital twins can reduce risks. Thanks to a thorough understanding of the population coverage, there is increased confidence that your device will work as expected. In other words, it reduces the risk of post-market device failure or, worse, product recall. Maybe even more importantly, it also informs you which types of patients are unsuitable for your device and have an increased risk that it will not work correctly.
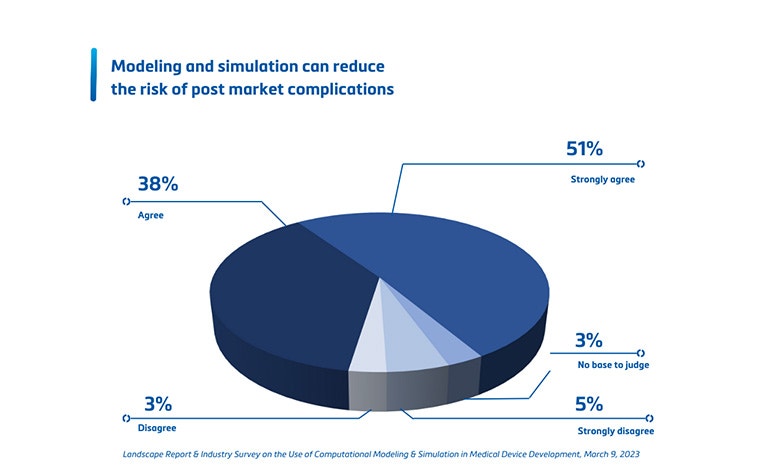

4. How to convince internal and external stakeholders?
Implementing digital twins as a standard device development tool is a disruptive change that requires convincing many stakeholders. Multiple internal decision makers need to understand what benefits it will bring to them. The most immediate groups to convince are the decision makers, leadership, and regulatory affairs in your company. They want to see how the use of digital twins will ultimately reduce the total R&D cost, the risk, and accelerate the commercial launch of a new solution by bringing a stronger case to regulatory bodies (key to convincing regulatory affairs). Use the methodology to calculate the ROI as explained above and apply it to your R&D project to bring this as a comprehensive case to your management. Another department that will typically need to be convinced is the purchasing department. They will be looking for an analysis of the investment (tools and costs) versus the ROI. A variation of that analysis will also be helpful for the IT department. For them, the analysis of the additional tools will not focus on the financial aspects but on what these tools entail (services, cloud, desktop, etc.) and the companies providing them (credibility, training, support, etc.). Essential to mention to all these internal stakeholders is that R&D based on digital twins is becoming the norm in medical device companies. You can refer to reference stories from other companies in our Virtual Patients e-book.
On the other hand, there are also external stakeholders to convince. For the investors and shareholders, the ROI will need to be highlighted. When working with key opinion leaders at specific hospitals, it is vital to get their buy-in early on. Pointing out the enhanced population coverage and how well it will anatomically fit the patients will help them understand the benefits and increase their confidence in using the innovative device. Lastly, regulatory bodies will also need to be convinced of the benefits of digital twins. Luckily, there is a trend that they are accepting that device development based on an enhanced understanding of the population leads to better devices and that including statistical population studies supports your case to regulatory bodies.

5. How to implement digital twins as a standard medical device development tool?
Integrating digital twins as a standard methodology in the medical device development process is an audacious goal that needs to be split into smaller chunks to make it manageable. Therefore, starting with just one R&D project first is advised. List the plan for this R&D project and ensure you know the benefits and limitations of digital twins (question 1). Then, identify if your project is suitable to apply Digital Twins on (question 2), and if needed, iterate until you define an appropriate project. As a next step, determine the return on investment (question 3) and plan to convince internal and external stakeholders (question 4). During this process, some additional questions might arise:
- What resources are needed? Are there specific skills required, and do we already have them in-house?
- What input data is needed? Do we already have these medical images, or is there an external source we can collaborate with?
- What tools are required, and do we already have access to these? What’s the total cost of this project (to compare it with the ROI)?
Based on the answers to these questions, you might have to define if an external partner is needed to provide you with the appropriate tools, knowledge, and services, depending on the level of in-house capabilities. Once these questions are answered, and a plan is defined, implement this technology gradually. Begin with only a few digital twins to better understand the anatomy and target population, continue a more extensive population study to define the relevant design input, and proceed to find extreme cases within the population analysis to validate your device… In this way, you gradually build your digital twin database and knowledge.
After this pilot project, take the time to evaluate at some intermediate points and at the end of the R&D project how digital twins helped to improve and accelerate this process. As this technology shift is very ambitious and can initially be intimidating, it is good to engage with experienced partners to support you. Use these results to convince internal and external stakeholders to continue using this technology. In this way, you can keep using the methodology of digital twins for each new suitable R&D project. Remember that you will add to the digital twin database each time and continue to strengthen your knowledge and expertise to benefit fully from digital twins.
L-103359-02
Share on:
You might also like
Never miss a story like this. Get curated content delivered straight to your inbox.
