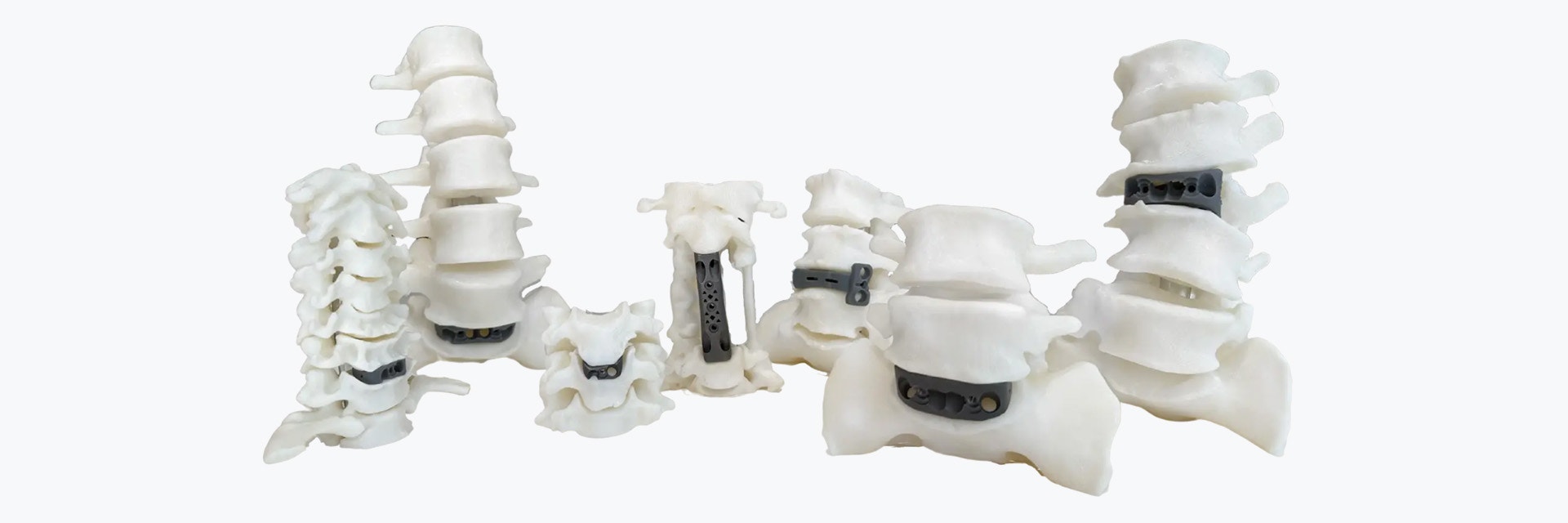
CUSTOMER STORY
Fusing Technology to Create Life-Changing Custom Spine Implants

We talked to Dr. William Parr, co-founder of 3DMorphic, an Australian start-up that designs and manufactures patient-specific spinal fusion devices via titanium 3D printing. With a combination of Materialise Mimics Core and their own in-house software and production, they are the only company that has a process that is fast enough to treat emergency trauma spinal patients with patient-specific implants. We cover why spinal surgery needs to get it right the first time, its processes, challenges, and what the future holds for this innovative start-up that shares our aim of mass personalization for better patient care.
A clear need for spinal fusion
Why did Dr. Parr focus on spinal fusion? As a postdoctoral researcher, he looked at spinal fusions for over five years and realized they weren’t working well. Dr. Parr explains, "Around 50% of spinal fusion patients have reoperations within ten years. Whereas, if you look at hip and knee, that's below 10% and getting better all the time. The need was clear."
Dr. Parr has been using Materialise Mimics for his research since 2009 and was well versed in using 3-matic to build very precise models of anatomy suitable for finite element analysis. So, when he co-founded the company in 2015, it just made sense: “I already knew the technology and liked it. And it just seemed obvious to me to use it going forward.”
The difference of a millimeter
Options for spine surgeons have increased dramatically in the past 20 years, but there is still room for improvement. As all spines are different, off-the-shelf spinal devices with generic shapes do not fit the anatomy well, resulting in movement. This movement prevents the patient from gaining immediate clinical benefits through joint stabilization following the surgery.
Long-term benefits of the surgery are only gained through spinal fusion and bone growth, which creates stabilization. And most importantly, if there’s too much movement, progenitor cells will differentiate into fibrous tissue instead of bone cells surrounding the implant. Once this happens, these cells can’t change back. As a result, bone fusion is impossible, and the procedure is at an increased risk of long-term failure.
Dr. Parr says, “It’s important when doing spinal fusion to get it right straight away and achieve immediate stability by using a device that fits the patient's anatomy. The device's proximity to the bone is important as bone can only bridge a one-to-two-millimeter gap, so the device surfaces need to be right next to the bone.” Once the gap between the device and the bone is bridged, the process of fusion starts. Longer-term fusion through the device happens over six months to a year. However, that can only occur after initial stability and fusion of the implant.
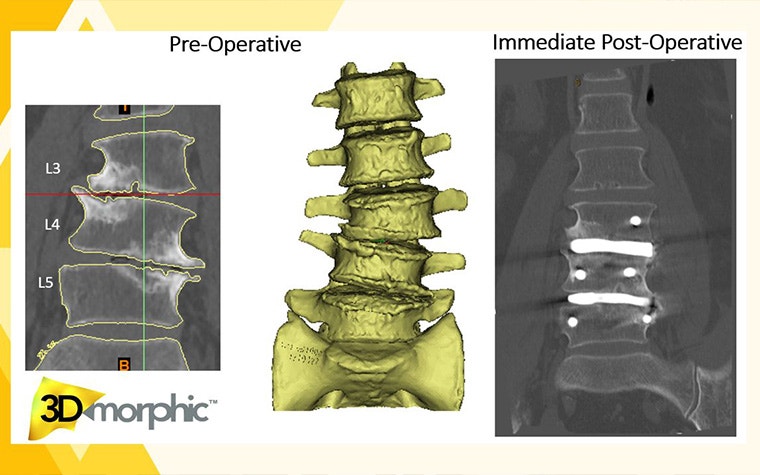

So how does it work?
Typically, the surgeon will diagnose the patient and assess the patient's suitability for spinal fusion. The surgeon then contacts 3DMorphic with the CT scan and requirements, and the team starts segmenting the 3D model of the patient’s anatomy using Mimics Core and designs the implant using their in-house software. “We then manufacture and post-process the devices in our vertically integrated production facility in Sydney before they’re delivered to the hospital, along with their associated instrument sets for the surgery. All the retraction, disc preparation, and implantation instruments are included,” adds Dr. Parr.
3DMorphic has also worked on emergency spinal procedures, which means the entire process — device design, manufacture, and arrival at the hospital for sterilization and implantation — has been completed within 24 – 48 hours of the surgeon ordering the device. Currently, they are the only company providing this service in Australia. In comparison, other companies offering patient-specific devices typically have a lead time of up to six weeks.
In an extreme case, the team has even gotten the process down to 9 hours.


Solutions for patients
Dr. Parr explains that their focus is on developing solutions that can radically change patients' lives for the better. He says, “I want to build technology where we would never have to say no to a patient in need.” Committed to this vision, he and his team maintain regular briefings weekly, during which they share ongoing projects and their impact, ensuring their work remains firmly rooted around the patients. Additionally, they cultivate a 'Wall of Happiness,' where they add patient stories to inspire their work.
Recently, 3DMorphic helped a 22-year-old man with hypermobility and Chiari malformation, which significantly impacted his quality of life. By the time six months had passed from the initial surgery, wherein conventional fusion techniques and implants were used, his body had reabsorbed the implanted bone graft. So, 3DMorphic worked with the surgeon to create custom-made fusion devices, and the patient improved considerably in both alignment and fusion within five months of the second surgery. Knowing the patient would have a better quality of life ahead of him was especially gratifying for the team.
A silver lining in regulatory and reimbursement pathways
The main challenges for 3DMorphic are common for anyone bringing new solutions to implantable medical devices — regulatory and reimbursement barriers that affect market access. The regulatory barriers are vital because it is essential to demonstrate the safety of the devices before they're implanted in people. Dr. Parr says, “It's a really long road to get from an innovative technology to this technology benefiting patients. Thankfully, the regulatory frameworks to deal with patient-specific devices are now emerging in Australia, the US, and Europe.” However, suitable reimbursement pathways that recognize the added value provided by the patient-specific approach and compensate for the additional costs are still needed.
New to the patient-specific device game? Self-assess for success
Dr. Parr offers advice to others hoping to start in a similar space: "Very rapidly try to assess whether your patient-specific solutions are addressing a real problem and significantly improving patient outcomes. If they are, it's important to establish the applicable regulatory and reimbursement pathways to know how your solutions will be regulated and how you will enter the market. If you don't establish these things very quickly, it will increase the business risk.”
He also thinks it's essential to self-assess whether forming a new company is the right move or whether your time and energy are better spent working for an existing company already in the space. He adds, “On the one hand, young companies can move fast and truly bring groundbreaking new technology to their field. But on the other hand, in the medical field, it is tough and costly to do.”
Bringing the future into the present
3DMorphic is going to continue to work on new technology to improve their production efficiency and introduce new and beneficial solutions and devices to the market. They want to expand their product range and continue to create more efficient systems in-house.
Dr. Parr explains, “People often say things like, ‘in the future…’ and ‘oh, this is the future…’, but the future will always remain just that, in the future unless it is pulled into the present, which is what we are trying to do for patients.”
L-103933-01
Materialise medical devices may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Materialise representative if you have questions about the availability of Materialise medical devices in your area.
Share on:
You might also like
Never miss a story like this. Get curated content delivered straight to your inbox.