Materialise CMF Standard+ Solutions
Empowering surgeons with a seamless, ready-to-use solutions
Apply a tailored, precise approach to every orthognathic, trauma, and reconstructive procedure in CMF surgery. These user-friendly modules include a comprehensive range of premium plates, screws, and instruments to provide you with the confidence and efficiency needed to deliver exceptional care.

Tailored precision for every CMF surgery
Reliable, versatile, and ready to use
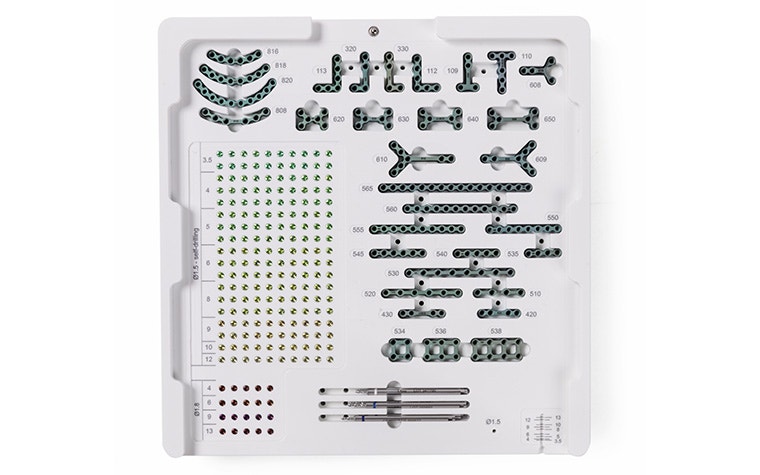
A comprehensive selection of standard plates and screws provides exactly what you need every time you enter the OR. Plates of various shapes, sizes, and thicknesses cover your diverse orthognathic, trauma, and reconstructive cases and your unique preferences.
Enhanced efficiency and traceability
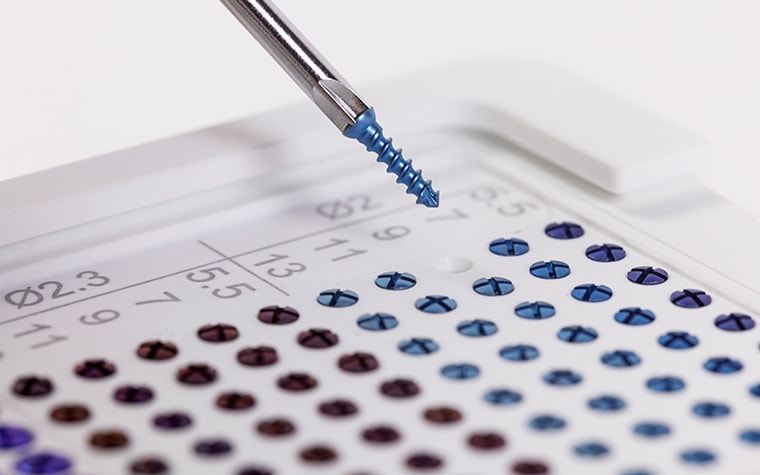
Easily identify and track the devices with clear color coding, and simplify your operations with a unified cruciform head. Their self-retentive design enables stable handling so your steady hand extends all the way to the tip of the screw.
Seamless integration with personalized solutions
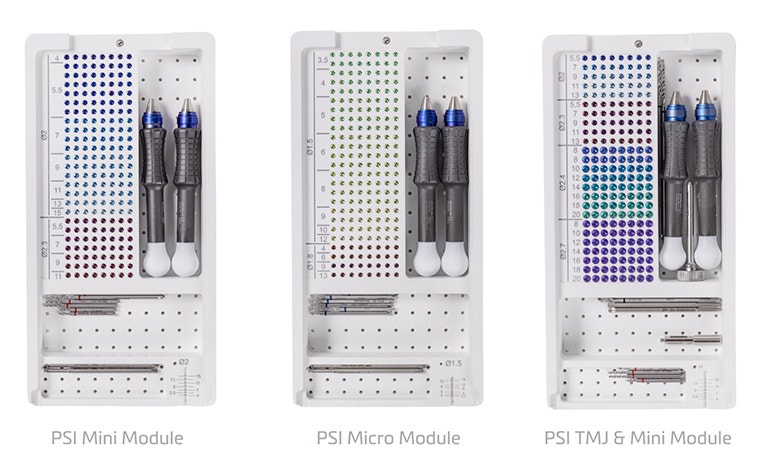
Experience a smooth combination of Materialise Standard+ Solutions and Materialise Personalized Solutions to eliminate compatibility issues and simplify operations.
See what's included
Explore the catalog
Discover our personalized CMF solutions
양악 수술
양악 스플린트, 3D 프린팅한 수술용 가이드 및 임플란트 등 양악 수술 전용의 다양한 맞춤형 솔루션을 통해 가장 복잡한 케이스의 요구 사항도 해결할 수 있습니다.
재건 수술
정확한 크기와 각도로 뼈 조각을 채취하고 정확한 위치에서 종양을 절제하기 위한 맞춤형 가이드입니다. 정밀한 재구성을 위한 맞춤형 플레이트와 적절한 나사.
트라우마
종양 조직 손실 및 외상으로 인한 조직 손실에 대한 맞춤형 솔루션을 제공합니다.
Frequently asked questions
Inspiration
Discover how others are benefiting from our CMF solutions.
L-101927-02