CASE STUDY
Innovative Methods for Aortic Aneurysm Device Testing
Medtronic uses the Mimics Innovation Suite to transform patient data into accurate and realistic physical test models for their stent designs and to quantify device performance.
The challenge
Developing test models that perform well in challenging conditions
Medtronic is the global leader in medical technology. The Endovascular division designs and manufactures devices that treat cardiovascular diseases such as thoracic and abdominal aortic aneurysms. Medtronic Endovascular commits unwaveringly to improving lives with patient outreach, educational programs that raise awareness of cardiovascular diseases, and the continuing pursuit of new treatment options. Medtronic Endovascular is proud to have helped physicians treat over 200,000 patients worldwide.


When testing grafts used to treat abdominal and thoracic aortic aneurysms, Medtronic’s goal is to develop models that help to accurately mimic in-vivo device performance. Due to the critical role these grafts play in a patient’s well-being, a new method of designing test models was developed. By incorporating statistical analysis with the development of benchtop test models, Medtronic is able to ensure that its devices will perform under challenging conditions.

The solution
Mimics Innovation Suite
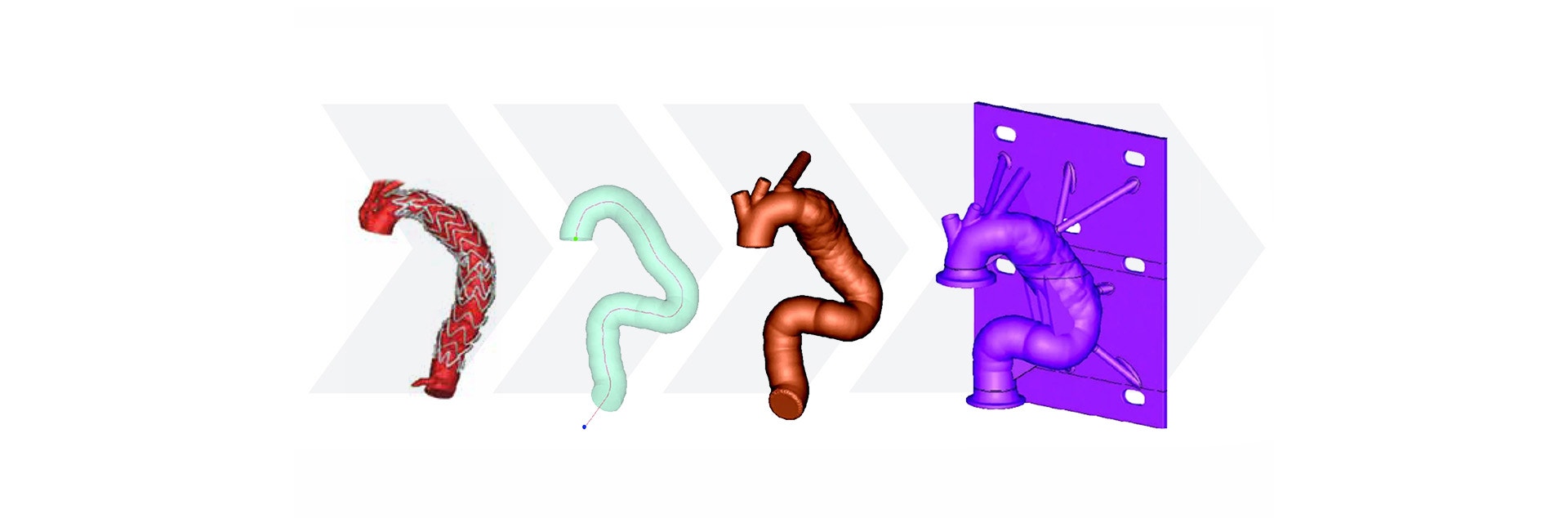
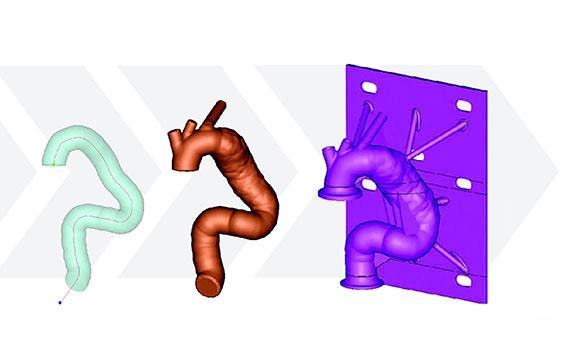
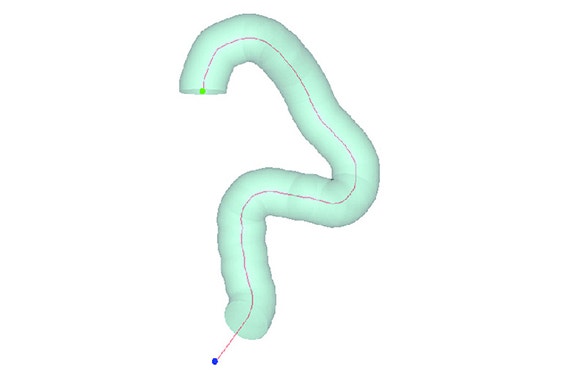
Using the Mimics Innovation Suite, Medtronic developed a method of obtaining geometric parameters from actual patient data to define the in-vivo use conditions. Patient CT data from the field was collected and delivered to the research and development team. Using Materialise Mimics Core software, the team segmented the 3D aortic model from the datasets. A centerline was automatically calculated in Mimics Core to fit the aortic model. To describe challenge-use conditions, the centerline was morphed to fit the 95th percentile value for each geometric parameter. Using this hybrid method of combining actual patient data and statistically assessed geometric parameters, the vascular models can be adapted to fit any requirements for testing purposes.
Using patient data to create benchtop models is important for accurate and realistic testing. “The Mimics Innovation Suite helped us to transform patient data into physical test models. Our stent designs were tested within the physical models to define and quantify device performance,” says Srinivasan Varahoor, Ph.D., Principal R&D Engineer at Medtronic Endovascular. The ability to utilize geometric parameters to quantify anatomy and show a method for developing a set of standardized, patient-based models using three-dimensional imaging and CAD tools has been a breakthrough for Medtronic Endovascular.
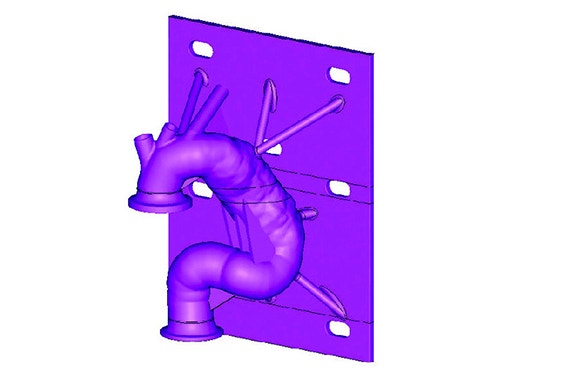
After forming the hybrid centerline, Materialise 3-matic was used to design a thin-walled patient-based aortic model. Supports and standard test fittings were also designed into the device before using 3D printing technology to print a physical benchtop test model.
The model was then fitted into the benchtop test apparatus with cycling fluid to evaluate and quantify several performance metrics of the stent graft and its delivery system.
Medtronic’s method for designing a patient-based benchtop test apparatus can be summarized in five steps:
- Measure geometric parameters
- Calculate centerline
- Adapt the centerline to fit a statistical model
- Design test apparatus
- Print the model with medical 3D printing technology
The result
Creating a new standard for benchtop testing


The use of the Mimics Innovation Suite to quantify anatomical geometry and generate a set of standard patient-based models has created an unsurpassed standard for benchtop testing at Medtronic. “Mimics Core allows us to quantify edge of failure conditions and 3-matic can incorporate those performance limits into next-generation device development test models,” says Srinivasan Varahoor. These models can help systematically pinpoint conditions for possible device failure during testing and, thereby, result in more robust designs. This approach is applicable to the testing and development of any vascular device system in the future.

The standard in engineering on anatomy
The Mimics Innovation Suite turns 3D image data into high-quality digital models in an accurate and efficient way. Starting from CT, MRI, or 3D ultrasound images, the Mimics Innovation Suite offers the most advanced image segmentation, the broadest anatomical measurement options, powerful CAD tools for engineering on anatomy and 3D printing, and accurate model preparation for FEA and CFD.
In this case study, the authors used the Mimics Innovation Suite to standardize their device deployment testing using the following steps:
- Transforming medical-image-based population data virtual benchtop model designs
- Exporting these virtual models in the required format for 3D printing
- Defining and quantifying device performance using novel population-based benchtop models
L-103928-01
Share on:
This case study in a few words
Healthcare
Mimics Innovation Suite
Patient-based medical devices