CASO DI STUDIO
Additive Manufacturing Helps MMI Go from Start-up to Microsurgery Instrumentation Success Story

If you haven’t heard of MMI, chances are you soon will. Having developed the world’s smallest wristed micro-instruments as well as tremor-reducing and motion scaling robotic technology for complex microsurgery procedures, the Italian company is pushing new boundaries for surgical performance and patient care.
What’s even more impressive is that the company only formed in 2015, yet by the end of 2019, their Symani Surgical System — incorporating the ground-breaking ‘NanoWrist’ technology — had already received its CE Mark in Europe. Since then, MMI’s NanoWrist Instruments have been successfully used in human cases in Italy. The company is now poised for commercialization across Europe with plans to pursue FDA clearance and expand to the United States.
Having the right team, funding, and technical engineering expertise has been vital to MMI’s rapid transformation from start-up to the microsurgery solution success story. But there is one more important ingredient that has helped speed up the scale-up.
“Where we are now would not have been possible,” explained Massimiliano Simi, Founder and Vice President of R&D at MMI, “without the use of additive manufacturing.”
Turning an R&D idea into a market-ready solution
MMI’s breakthrough came in 2017 when, after a year and a half of technical development, the company announced its first milestone: the world’s smallest robotic wrist, 3 mm in diameter and with tips just 150 microns in width. Giving microsurgeons the dexterity and precision to carry out complex and hugely delicate reconstructive small anatomy procedures, it wasn’t long before MMI attracted investment. It was time to move from R&D prototyping to fine-tuning for commercially viable production.
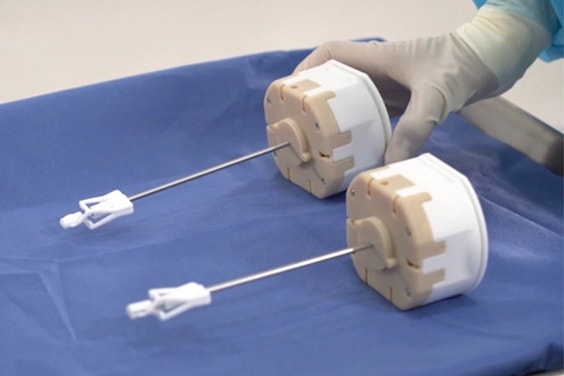

With AM, MMI can incorporate interior channels and integrate multiple components into one to improve performance.
“Working with Materialise to leverage the benefits of additive manufacturing (AM) has allowed us to really fast track design iterations on a number of components critical to the development of our microsurgery system, helping us move from prototyping to product in a much shorter time frame than would otherwise be possible,” said Massimiliano. “For a project like ours, this is hugely important as we need the adaptability to keep developing. With micro-instrumentation, an adjustment of just a 100th of a millimeter can have a significant impact on performance. Where switching molds for each new iteration would become incredibly costly, 3D printing allows us to be nimble with our design iterations.”
Today MMI uses AM extensively to manufacture end-use parts for their surgical kits, particularly disposable parts of instruments. Besides the ability to manufacture on demand, MMI’s innovation-minded product team also appreciates 3D printing’s suitability for complex design. The parts manufactured with AM are designed with the technology in mind, incorporating interior channels and integrating multiple components into one to improve performance.


“We had to think about not only improving functionality but also usability. Design can be the key to minimizing operator error,” explained Massimiliano.
AM applied to single-use sterile instrumentation
As well as providing a booster rocket for R&D, AM has also given MMI a time-critical answer to an end-part challenge.
Giancarlo Testaverde, Vice President of Operations at MMI, commented: “Our solution necessitates sterile, disposable instrumentation for each case. As part of our path to market, we needed to produce enough components to validate our solution and process. To optimize performance, a very particular shape is also required with tolerances hard to produce through molding — certainly not economical for the volume we are talking about right now.
“The stability, performance, and biocompatibility of materials and the qualified 3D printing process allow us to create the unusual structure required for optimal performance. This has been ideal for our purposes at this stage of our growth strategy. We needed to prove performance quickly and have been able to do so.”
The perfect partner for re-invention
Having now reached a pivotal point in its development as a business, with the Symani microsurgery system poised for wider usage globally and requiring increased production volumes, MMI is keen to further its relationship with AM.
“AM, and working with Materialise as a supply chain partner, has given us two important advantages so far in our journey. The flexibility to adjust design rapidly and the confidence of end-part performance in a ‘market-ready’ context. ”
— Massimiliano Simi, Founder and Vice President of R&D at MMI


“As we scale up, it may be that we switch to an injection molding solution for end-use components currently produced using AM but if so, we’ll be investing in the equipment and processes needed knowing that the end result is market-proven. AM has given us that basis.
“Alternatively, AM may scale with us. The supply responsiveness and quality delivered certainly meet our immediate requirements. By having this technology in our development and manufacturing toolkit, we have options. That’s hugely valuable for us right now.”
Giancarlo added: “Yes, the nature of our technical focus and the microsurgery sector we serve means that development can, and should, never stop. Continuous reinvention and refinement is part of our DNA in this respect, which is why our relationship with AM and in particular, Materialise, will continue to grow.”
Condividi su:
Il caso di studio in breve
MMI
MedTech
Serial additive manufacturing
Rapid design iteration
Design freedom
Fast time-to-market
