XR for Mimics Viewer
Extended reality (XR) can give you a more immersive and intuitive way to interact with medical images. XR includes augmented reality (AR), virtual reality (VR), and mixed reality (MR). Complement your 3D printing activities with an accessible environment to scale up your 3D services toward clinical applications. Ready to discover XR functionality in Mimics Viewer?
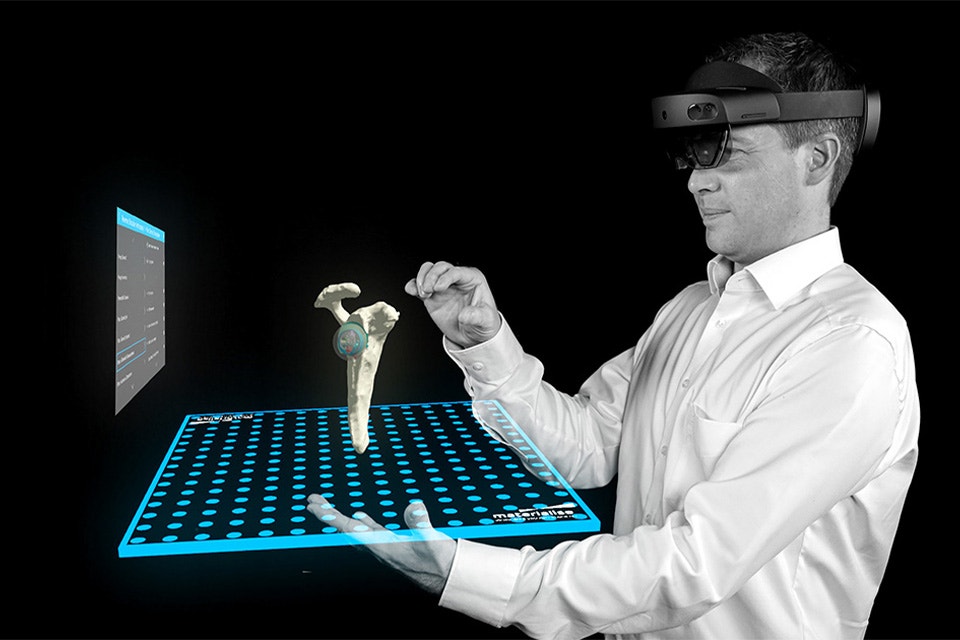
Benefits
Flexible
More versatile than 3D-printed models through visualization of measurements, zooming, scaling, etc
Time saving
Shorten the lead time for time critical applications like trauma by giving surgeons immediate access to the 3D model
Cost efficient
XR is more affordable than 3D printing — allowing you to scale up your activities
Accessible
Makes it easier to give your clinicians access to your 3D models in XR
Give your clinicians access to your 3D model in XR:
- Easily transfer your segmentation to XR visualization while staying connected to your workflow
- Keep the learning curve for your physician low with a more intuitive process compared to other software
- Visualize your Mimics Viewer cases on the most known and available market standard devices — Microsoft HoloLens 2 (AR), Magic Leap 2 (AR), or Meta Quest 2 and 3 (AR/VR)
- Use medically cleared* software for quality assurance
- Integrated with different Materialise software, all provided in one eco-system
See the AR feature in action
Get inspired
Learn how XR can guide you with new ways to visualize procedures and how XR's possibilities extend even further.
*Now cleared for medical usage in the EU and in the US. Materialise medical devices may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets.
Please contact your Materialise representative if you have questions about the availability of Materialise medical devices in your area.
L-103317-02


