TRENDS
State of the Healthcare Market 2020: Point-of-Care 3D Printing Trends
The adoption of 3D printing by hospitals and clinics continues to grow at a rapid pace. The technology is becoming more accessible and the benefits to physicians and patients are better understood. So more hospitals are investing in 3D printing infrastructure as an enabler of personalized healthcare. We assess the 4 market highlights from 2019 and take a look ahead to 2020.
Point-of-care 3D printing refers to the use of 3D printing within the hospital setting, near the place where clinical care is delivered. The most common application is the creation of anatomical models from patient imaging (CT or MRI). These can be used as a tool for pre-procedural planning, communicating with patients, and simulating training exercises. This yields benefits for all stakeholders including the patient, care providers, and the healthcare facility.
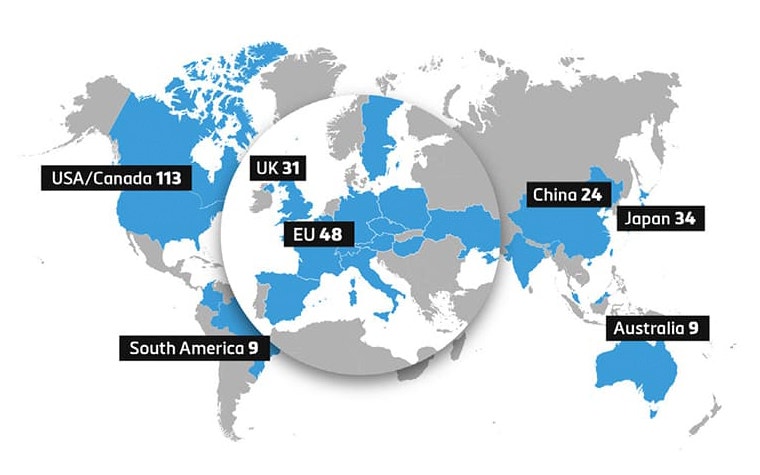

We continue to see a growing number of hospitals make initial investments to launch 3D printing programs. Other hospitals are investing in the growth of their facilities as the benefits are understood and demand increases — both in procedural volume and breadth of applications. This is demonstrated on a global scale as seen in Figure 1, with markets in Europe and Asia showing growth in 3D printing adoption.
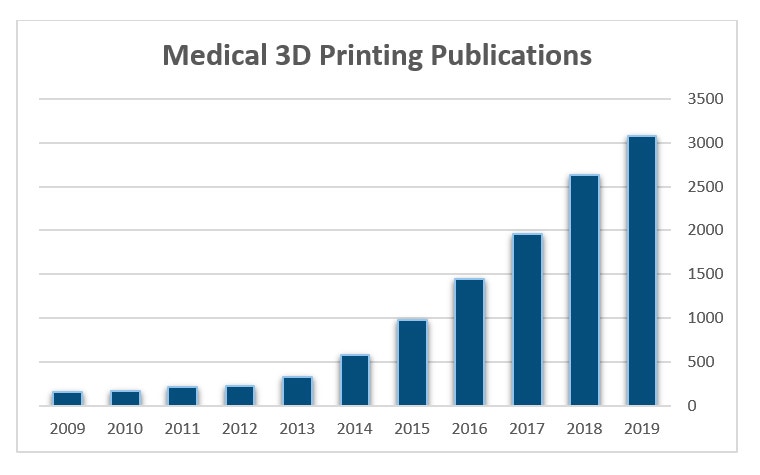
Scientific evidence supporting the clinical and financial benefits of using 3D printing at point-of-care is also increasing.. In 2019, articles available on Pubmed with reference to 3D printing reached a new high, as seen below in Figure 2. The body of evidence is growing with a recent review article published by Ballard et al. reporting average operating room time savings of 62 minutes per case and attributed cost savings of $3,720 per case when 3D-printed anatomical models are used to prepare for orthopaedic and maxillofacial surgery1.

4 market highlights from 2019
1. Billing codes in the U.S. implemented
Category III CPT billing codes are now available for 3D printing in the United States. Codes 0559T and 0569T are available for individually prepared anatomic models. Additional codes 0561T and 0562T, are used for 3D-printed anatomic guides derived from patient imaging data. In a first phase, the codes will not come with guarantee of payment, but will serve to collect necessary data from the market to further reimbursement possibilities for 3D printing in the clinic.
2. 3D printing clinical data registry announced by the Radiological Society of North America (RSNA) and the American College of Radiology (ACR)
Leading radiology societies announced a new registry to collect data to understand clinical benefits and appropriate use of 3D printing. Materialise along with partners Formlabs, Stratasys, and HP provided unrestricted educational grants to financially support the registry.
"Medical models and surgical guides have been 3D printed for well over a decade, as niche applications — and without CPT codes. For example, craniomaxillofacial care providers generally accept that 3D printing is valuable and integral to patient care," said Frank Rybicki, MD, PhD, FACR, chair of the ACR Committee on Appropriateness Criteria and founding chair of the RSNA 3D Printing Special Interest Group.
“However, when applying for CPT codes, it became clear that this ‘general acceptance’ lacked peer-reviewed literature to demonstrate value. This registry will supply data to benchmark the value of this subspecialty.”
3. New 3D printing hardware and materials for anatomic modeling
Materialise completed projects with both HP and Formlabs to certify new printers and materials as compatible with the FDA-cleared Materialise Mimics software. HP enables full-color 3D printing with the new JF 580 printer while Formlabs released a new iteration of their popular desktop using stereolithography technology with the Form3 and Form3B.

4. Medical societies experience growth in 3D interest among members
Since the founding of the RSNA 3D Printing Special Interest Group (SIG) by Dr. Frank Rybicki in 2015, membership has steadily increased. Now more than 500 international representatives contribute to the advancement of the field. More recently, the Society for Cardiac Magnetic Resonance (SCMR) has established a similar working group of cardiac imagers who also seek to support the growth of 3D technology used clinically.
4 things to look for in 2020
1. The year of the COVID-19 crisis
The COVID-19 crisis is expected to play out for much of the year 2020, placing stress on healthcare systems around the globe. Hospitals supporting 3D Printing labs will take the opportunity to repurpose these resources to aid in the crisis. Labs traditionally printing anatomical models for surgical planning will shift focus to address expected shortages of critical safety and life-supporting equipment.
2. Further integration of 3D printing in the clinical workflow
The new DICOM encapsulated STL standard enables 3D printing files to be easily archived in hospital PACS and affiliated with patients’ medical records. The new DICOM standard will be available in Mimics 23, which is releasing this year. We expect to see more widespread adoption of these standards.
3. Growth in the use of extended reality devices
Augmented and virtual reality technologies will continue to be explored as an adjunct to 3D printing applications. Initially they may be used as a visualization and training aide but ultimately they will support procedural planning and intra-operative guidance. Advances in hardware combined with targeted software applications will fuel the growth.
According to Jesse Courtier, Pediatric Radiologist at University of California, San Francisco: “I believe AR complements 3D printing by allowing for rapid prototyping and iterative model improvement in an economical and environmentally friendly way. Models can be repeatedly tested and viewed at full anatomical scale without regard to some of the constraints of physical models (gravity, thickness, printer size, cost). It also allows for remote collaboration through the sharing of 3D models with colleagues without the need for physical modeling.”
“I believe AR complements 3D printing by allowing for rapid prototyping and iterative model improvement in an economical and environmentally friendly way. ”
— Dr. Jesse Courtier, Pediatric Radiologist at the University of California, San Francisco
4. Additional regulatory clarity in the European Union and U.S.
The impact of the new E.U. Medical Device Regulations on point-of-care manufacturers is uncertain. In the U.S., the F.D.A. continues to support a dialogue with stakeholders to determine appropriate guidance for safe and effective use of 3D printing in a point-of-care setting. 2020 will be a year where regulations related to medical 3D printing become clearer.
References
1. Ballard et al., Medical 3D printing cost-savings in Orthopaedic and Maxillofacial Surgery, Academic Radiology, 2019.
2. Khan, Rybicki & Weadock, RSNA and ACR to Collaborate on Landmark Medical 3D Printing Registry, https://www.rsna.org/en/news/2019/August/3D-Printing-Registry, 2019.
L-101080-01
Share on:
You might also like
Never miss a story like this. Get curated content delivered straight to your inbox.
